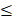SAN DIEGO, CA / ACCESS Newswire / November 20, 2025 / Revelation Biosciences, Inc. (NASDAQ:REVB) (the “Company” or “Revelation”), a clinical-stage life sciences company that is focused on rebalancing inflammation, today announced successful submission and acceptance of the end-of-phase 1 meeting package to FDA and that the company is on track to hold the meeting later this year. The primary purpose of this meeting is to establish agency feedback and input into the clinical development and regulatory approval pathway for Gemini as a treatment for acute kidney injury (AKI).
“The Revelation team has worked tirelessly on our end-of-phase 1 meeting submission, and we look forward to our FDA meeting scheduled for later this year,” said James Rolke, Chief Executive Officer of Revelation. “Our near-term focus will be conducting the necessary activities for initiating and running a later stage clinical study during 2026 to evaluate the ability of Gemini to effectively treat AKI. In addition, we plan to publish additional positive results from the PRIME study that completed this year and to expand potential uses of Gemini for other conditions through preclinical and clinical testing.”
Revelation recently announced positive safety and activity data from its Phase 1b PRIME clinical study in stage 3 and 4 chronic kidney disease patients. The primary endpoint to evaluate the safety and tolerability of escalating doses of Gemini was met. In addition, the potential of Gemini to treat acute and chronic inflammatory conditions was demonstrated by significantly reducing inflammatory activity and restoring normal cellular response to stimuli at the cellular level, as measured in peripheral blood mononuclear cells (PBMCs) isolated from patients at predose, 2, 24, and 168 hours post-dose.
About the PRIME Study
The PRIME study enrolled 40 patients from 32 to 78 years of age, at 3 US-based clinics specializing in the care of patients with CKD. A total of 5 cohorts (8 per cohort, 6 treated/2 placebo) at 4 dose levels were enrolled: a subtherapeutic dose, a low dose, the target dose (cohorts 3 and 4), and a high dose. Additionally, an extension protocol was conducted to collect PBMC and biomarker samples in 8 Gemini naive or secondary naive patients. The primary endpoint evaluation of safety was met. Patient PBMCs were isolated predose and at 2, 24, and 168 hours post-dose. PBMCs were analyzed ex vivo for background inflammation by measurement of IL-1β, TNF-α, IL-6, IL-10, and IL‑1RA. Cells were also assessed for response to stimulation by lipopolysaccharide (LPS, also known as endotoxin) or high mobility group box-1 protein (HMGB1). Subgroup analysis divided patients into two categories, those with PBMCs of minimal background inflammation activity (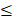 40 pg/mL IL-1β) and normal response to stimuli, and those with significant background inflammation activity (>40 pg/mL IL-1β). Approximately 50% of patients were in each group.
40 pg/mL IL-1β) and normal response to stimuli, and those with significant background inflammation activity (>40 pg/mL IL-1β). Approximately 50% of patients were in each group.
In patients with high background PBMC activity, Gemini significantly reduced inflammation relative to placebo patient PBMCs at all time-points post dose (IL-1β: p<0.01, IL-6: p<0.01, TNF-α: p=0.05, IL-10: p<0.01, IL-1RA: p<0.001). Background inflammation was reduced to levels comparable to PBMCs isolated from healthy subjects. In addition, Gemini was able to demonstrate correction of the immunoparalysis typical to chronic disease. Gemini significantly increased the responsiveness to LPS stimulation in high background patient PBMCs relative to placebo at all timepoints (IL-1β: p<0.0001, IL-6: p<0.0002, TNF-α: p<0.002, IL-10: p=0.09, IL-1RA: p<0.01). Gemini also significantly increased the responsiveness of patient PBMCs with high background vs placebo patient PBMCs with high background at all time-points to HMGB1 stimulation (IL-1β: p<0.05, IL-6: p<0.01, TNF-α: p<0.01, IL-10: p<0.05, IL-1RA: p<0.002). The increased responsiveness was comparable to PBMCs isolated from healthy subjects. These results show the ability of Gemini to salvage the normal inflammatory response, even as far as one week after a single dose. For the low background patients, as expected, Gemini does not increase inflammatory activity. Additional analysis on the effect of LPS or HMGB-1 stimulation is ongoing.
Revelation will seek to publish these results and those from ongoing additional data analysis. For more information on Revelation, please visit www.RevBiosciences.com.
About AKI
Acute Kidney Injury or AKI, also known as acute renal failure, is defined as a rapid loss of kidney function. AKI causes a build-up of waste products in blood and makes it more difficult for kidneys to maintain the correct balance of fluid in the body. AKI can also significantly impact other organs such as the brain, heart, and lungs. Severe AKI requiring dialysis significantly increases the likelihood of worse outcomes including longer time in an ICU, potential to develop chronic kidney disease and death.
AKI is a major cause of morbidity and mortality, affecting more than 10% of all hospitalized patients and more than 50% of patients admitted to intensive care units. Renal replacement therapy (dialysis) is still the only therapeutic option in the treatment of the consequences of severe AKI and is required in approximately 20% of all critically ill patients. Despite the fact that these patients show high mortality rates, up to 40% of patients who survive such an episode have develop chronic kidney disease or end-stage renal disease. As such, new therapies to treat AKI are currently needed.
About Gemini
Gemini is the Company’s proprietary formulation of phosphorylated hexaacyl disaccharide (PHAD®), a toll-like receptor 4 (TLR4) agonist. TLR4 stimulation with Gemini rebalances the innate immune response and has been demonstrated to have the potential to treat acute and chronic diseases associated with dysregulated inflammation. Gemini is currently being evaluated as a potential treatment for acute kidney injury (GEMINI-AKI program). Gemini is also being developed as a treatment for chronic kidney disease (GEMINI-CKD program), as a treatment to reduce hyperinflammation and infection associated with severe burn (GEM-PBI) and as a treatment to prevent post-surgical infection (GEMINI-PSI program). The potential of Gemini has been demonstrated in multiple preclinical models of AKI, CKD and infection, as well as in two phase 1 clinical studies. See additional detail here.
About Revelation Biosciences, Inc.
Revelation Biosciences, Inc. is a clinical stage life sciences company focused on rebalancing inflammation using its proprietary formulation Gemini. Revelation has multiple ongoing programs to evaluate Gemini, including the treatment of chronic kidney disease, prevention for post-surgical infection and as a treatment for acute kidney injury.
For more information on Revelation, please visit www.RevBiosciences.com.
Forward-Looking Statements
This press release contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking statements are statements that are not historical facts. These forward-looking statements are generally identified by the words “anticipate”, “believe”, “expect”, “estimate”, “plan”, “outlook”, and “project” and other similar expressions. We caution investors that forward-looking statements are based on management’s expectations and are only predictions or statements of current expectations and involve known and unknown risks, uncertainties and other factors that may cause actual results to be materially different from those anticipated by the forward-looking statements. Revelation cautions readers not to place undue reliance on any such forward looking statements, which speak only as of the date they were made. The following factors, among others, could cause actual results to differ materially from those described in these forward-looking statements: the ability of Revelation to meet its financial and strategic goals, due to, among other things, competition; the ability of Revelation to grow and manage growth profitability and retain its key employees; the possibility that the Revelation may be adversely affected by other economic, business, and/or competitive factors; risks relating to the successful development of Revelation’s product candidates; the ability to successfully complete planned clinical studies of its product candidates; the risk that we may not fully enroll our clinical studies or enrollment will take longer than expected; risks relating to the occurrence of adverse safety events and/or unexpected concerns that may arise from data or analysis from our clinical studies; changes in applicable laws or regulations; expected initiation of the clinical studies, the timing of clinical data; the outcome of the clinical data, including whether the results of such study is positive or whether it can be replicated; the outcome of data collected, including whether the results of such data and/or correlation can be replicated; the timing, costs, conduct and outcome of our other clinical studies; the anticipated treatment of future clinical data by the FDA, the EMA or other regulatory authorities, including whether such data will be sufficient for approval; the success of future development activities for its product candidates; potential indications for which product candidates may be developed; the ability of Revelation to maintain the listing of its securities on NASDAQ; the expected duration over which Revelation’s balances will fund its operations; and other risks and uncertainties described herein, as well as those risks and uncertainties discussed from time to time in other reports and other public filings with the SEC by Revelation.
Company Contact
Mike Porter
Investor Relations
Porter LaVay & Rose Inc.
Email: mike@plrinvest.com
Chester Zygmont, III
Chief Financial Officer
Revelation Biosciences Inc.
Email: czygmont@revbiosciences.com
SOURCE: Revelation Biosciences, Inc.
View the original press release on ACCESS Newswire